In principle, it is necessary to take the same procedures as the drug registration, but the required time and procedure are comparatively short and simple.
Foreign pre-clinical and clinical data, in principle is accepted with some exceptions. It is, however, faster and less expensive to produce some pre-clinical data in Japan.
MBS has a wide range of product registration experience; ranging from disposable products to medical electronic products.
In order to extend the life cycle of your product upon completion of product registration in abroad you should start preparation of the product registration with the Japanese MHLW. MBS will assist in the procedures marked with your company.
MBS mission
D-MAH
- Pilot to Restrictive Approval of Medical Equipment in Foreign Countries
- Application to the Ministry of Health, Labour and Welfare
- Protect classified product's information
- Control distributor
MAH
- Distribution and sales
- Logistics
- Storage I Stock management
- Traceability control
- Pharmaceutical category full support service
- Maintenance support
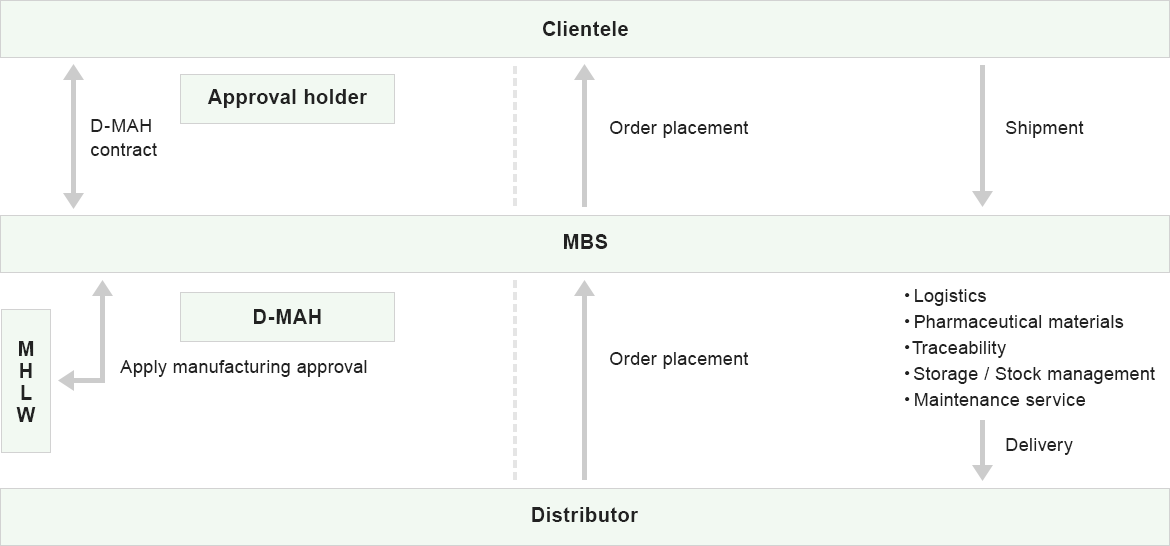
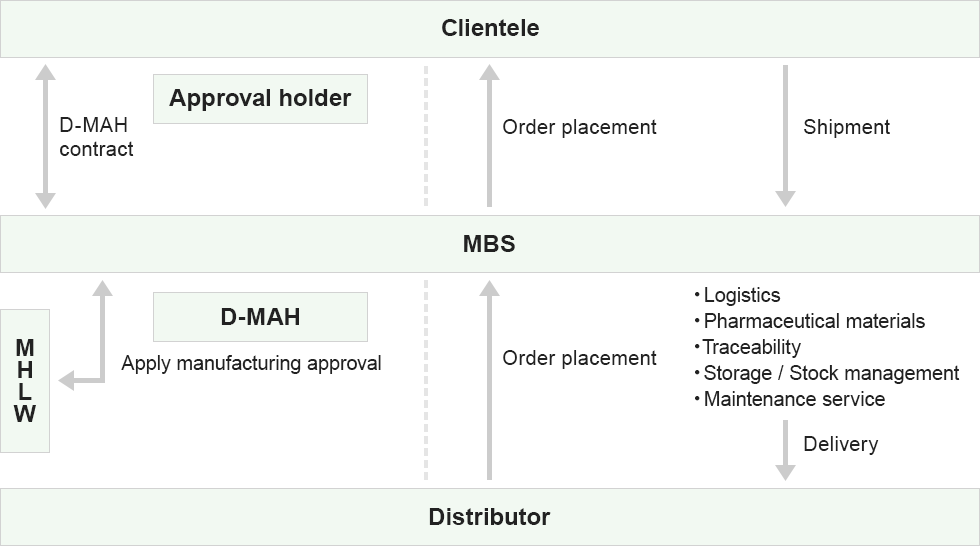
MBS total operation
MBS associate with foreign equipment manufacturer to offer full support consulting service who wish to develop their business in USA, EU countries, Korea, China, Taiwan and Australia.


